The Royal Institution
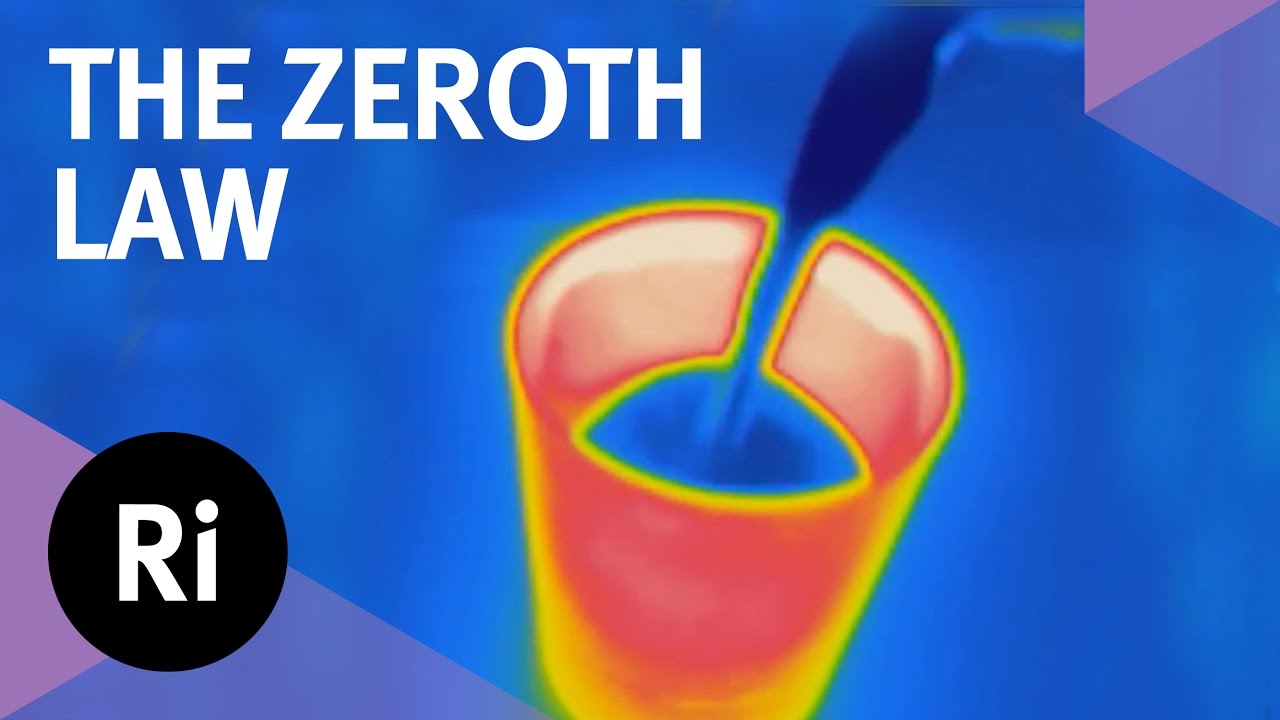
Why is there a zeroth law of thermodynamics? What use is such a simple-sounding law? And how can it be used to smash glass? Chemical engineer Valeska Ting explains in the first film from our 2016 advent calendar, all about thermodynamics.
Visit the online advent calendar: http://www.rigb.org/advent?utm_source=youtube&utm_medium=social&utm_campaign=201612_channel_advent
Subscribe for regular science videos: http://bit.ly/RiSubscRibe
The first, second and third laws of thermodynamics get all the glory. They’re the most well known and frequently mentioned. But underpinning them all is a final law so fundamental that, although it was established last, had to be moved to the front of the list: the zeroth law. In the first film of our 2016 advent calendar, chemical engineer Valeska Ting explores the zeroth law of thermodynamics.
The zeroth law is essentially an observation: if two systems are both in thermal equilibrium with a third, they are also in equilibrium with each other. This seemingly simple mantra is essential to our concept of temperature, as Valeska, armed with some very hot glasses, explains.
Our 2016 advent calendar explores the four laws of thermodynamics through 24 short films, released daily in the run up to Christmas. We’ll have explosive demonstrations, unique animations and even a musical number. Sign up to receive each instalment by email from rigb.org/advent
The Ri is on Twitter: http://twitter.com/ri_science
and Facebook: http://www.facebook.com/royalinstitution
and Tumblr: http://ri-science.tumblr.com/
Our editorial policy: http://www.rigb.org/home/editorial-policy
Subscribe for the latest science videos: http://bit.ly/RiNewsletter
Source


Possibly difficulty coast absence reduce skirt diversity significance generous celebration.
Justice is not done to this topic by this Ri video. A physics text book like "Resnick and Halliday" explains way better why this zeroth law is fundamental and not so obvious.
English isn't my mine, can someone give me the point of this video, please :v ?
I think to properly motivate this zeroth law you need to start at a more basic level. Maybe explain what exactly thermal equilibrium means, derive the notion of temperature and establish there is no a priori transitivity. Even though this is meant for non-experts, it just doesn’t make sense without properly explaining the underlying model. You can see the resulting discussion in many of the comments.
mLaw (Mikes Law) if a 4th glass is added, and it is not at equilibrium, then N4 != N1-3
Zeroth Law kinda feels like he got an everyone wins medal, unless, there is more I am missing, thus, missing from this video
Valeska Ting you are lovely.
What would it look like if you used thermal imaging in a 3-D camera technology?
the girl speaks perfect English with British accent
@The_Royal_Institution I'm wondering: Does the zeroth law also remain valid if relativistic effects are taken into account, say, if one of the glasses is traveling near the speed of light?
Thermydonamics. Now you cannot unhear.
a clever kiwi
Go Kiwi!
Quantum mechanic does not say so
what is the music?
Is this not common knowledge? I'm seriously asking.
wait…
1) Isn't this just basic logic? (https://en.wikipedia.org/wiki/Transitive_relation)
(A=B ^ B=C) => (A=C)?
2) Was Celsius into lipstick? not judging here 🙂
a = b
b = c
so a = c
why is there a law on this ….. 5 year olds proably know this aready
that's a rather boring example. this is a much more interesting example. imagine you're having and menage a trois, a threesome, with two girls and a guy. if the two girls are each in thermal equilibrium with the guy then the two girls will also be in thermal equilibrium with each other 🙂
BUT WHY????? Does every object with a temperature above 0K emit infrared frequency radiotion???!!!
Brilliant to see someone who looks so happy and excited to be talking about science.
this is a clear, concise explanation of the 4 laws. I will use this as a model for my class and i hope you will produce new presentation..
Zeroth lawIf A is a friend of B and B is a friend of C then A is also friend of C 😱😱😱
question…is it not wrong for scientists to tell the peoples that time only began when the baby was born in the big bang………………………………..when in fact it is not improbable that our universe is nothing more than a new time zone in a much older universe and that the steady state universe may well exist far beyond our capability to detect whats out there. that the steady state and big bang universe can be unified in theory.
I really tire of people mocking the traditional system with such colorful adjectives. Its called "pretentiousness". The Fahrenheit scale was not decided upon "arbitrarily", so dont use that word to contrast the two. Anyone with half a brain can look up the basis of the scale. You want to talk about idiocy? Mr. Celsius originally defined 100 as freezing and 0 as boiling.
How exactly does "transitivity" make way for measurability? There is a huge leap in logic here. I think an explanation or some elaboration is in order.
I think I'm missing the point, but what you said about if A=C and B=C, then A=B… I was just sat here thinking "no shit"… am I missing the point? It just seems obvious
Galileo did invent a thermometer, called Galileo's air thermometer (more accurately termed a thermoscope), in or before 1603
You missed the subtle point of the 0th law. Temperature is a state function based on the nature of heat itself and temperature is its independent measure regardless of it source. So for objects A, B and C (not necessarily identical) if A is heated by microwaves to temperature T, B heated by chemical reaction to temperature T, and C heated by the sun to temperature T – the heat in A, B and C is the same in the sense of summed atomic motion (but not the total amount of heat for different objects) independent of the heat source. Subtle but necessary for the other laws to work.
U r very beautiful
I love your accent
She's so cute, it hurts!
Mr. Celsius actually created the temperature scale with zero as the water boiling point and 100 as freezing!.
what temperature do the thermometers start at?
Merci beaucoup! We now have French subtitles for this video. If you too would like to try your hand in translating some science into your native language you can follow the link here – https://www.youtube.com/timedtext_video?ref=wt&v=PE_zpk-EznQ&auto=yes&bl=watch
I found a good source to master my basics!!! Thank u😎😎
https://www.youtube.com/watch?v=t8Nm9bnWOTs his track record speak for it self but i am sketish. initially from someone in chemestry and physic ( Caltech) Tolman, Richard C. (1917).
“The Measurable Quantities
of Physics”
.
Phys. Rev
.
9
(3): 237–253 it is mentionned all over the web but couldnt find one place the article of physic review was existing . only reference , no article . there is the other guy dr Robitaille mention but that is 1961 way way later ! would you per chance have data of this or another since if i recall you did invite this guy at the royal whatsnot!( whatsnot beign the exact name of that time since name change!
isnt the thermometer calibrated with gravity in mind? wouldnt that be an issue ( given that gravity laymen exemple of its existance have yet to be born?)
since most things in universe work via delta( no delta = death) its safe to say a lot of assumption are useless in the real world, unless they are known. applying this on unknown? i would ratter assume there is a delta , cause its very rare that something happen without a type of delta of a sort! how i make this work? simple: nothing( infinite) want to be filled ( you can replace the word with something that convey similar intention since there are likely a lot of variant) by something ( finite)! something( finite) want to fill nothing( infinite) simple hey ! applicable to all of periodic table and likely also alchemy table! so this means that you cant ask nothing ( infinite) to fill something( finite) its just impossible! so what does this mean in real world on the periodic table? lets take bismuth and hydrogen! lets use the electron value. they have similar electron count . closer to nothing . lets use the same 2 exemple but this time for weight! hydrogen still is closer to nothing ( want to be filled) but bismuth weight is closer to something ( want to fill nothing) easy right! and no , this isnt from me, nikolas tesla worked via this daily in his electrical experiement . like the a/c motor . he mentionned in some paper and it ended up in a free for all thing called my invention from nikolas tesla . but if you read between the line , he does think that the bigest delta possible is always very close to nothing. his brush motor made use of this initially ( before he came up with the brushless version)
Do the laws of thermodynamics apply to all forms of energy or only energy in the form of heat?
For example, if I raise a one pound weight up one foot in the Earth's gravitational field, it now has one foot-pound more of potential energy than it did before I raised it. Did it get warmer or colder or stay the same temperature? I'm confused as to how thermodynamic principals apply in this case.
I think I have a crush on her.
يالها من إبتسامة
What a smile
How about the proposed fourth law which states that thermodynamic equations must be balanced regarding their extrinsic and intrinsic properties?
The thermometer shouldn't touch the bottom and corner of the vessel. 🙄🙄🙄🙄🙄
There are actually five laws of thermodynamics…the fifth having to do with 'laws' regarding interactive properties of extensive and intensive properties.